|
|
Time Series Analysis:Leptospirosis |
1.Leptospirosis
Leptospirosis is an infectious disease that affects human as well as animals. It is also a zoonotic disease, which becomes a public health problem throughout the world. Leptospirosis is caused by infection with pathogenic spirochete bacteria of the family Leptospiraceae and genus Leptospira interrogans. There are over 250 serovars of this bacterium that can be identified by the Microscopic Agglutination Test (MAT). The size of leptospires is about 0.1-0.2 μm in diameter and 6-20 μm in length.
This disease was first reported in 1886 by Weil, and therefore called the classical Weil’s disease. For Thailand, this disease was first reported in 1942 by Yunibandhu, who noted the Weil’s syndrome. Subsequently, leptospirosis was added to the list of diseases in the National Disease Surveillance System in Ministry of Public Health.
The symptoms of this disease may range from mild, flu-like illness to the fatal (ictero) hemorrhagic form, involving renal and/or liver failure. The renal failure may occur for 7-10 days. An antibiotic therapy is recommended for clinical treatment only during the initial phase of illness using doxycycline, penicillin, ampicillin, and amoxicillin etc. For severe leptospirosis or late administration of antibiotics, only dialysis was shown to be effective.
The transmission can be either direct via urine of the carrier mammals or indirect contact via contamination of soil or water. The infection in human may be occupational associated in most cases due to direct contact with contagious material by farmers, veterinarians or others. Indirect contact may occur with sewage workers, miners, fish farmers, and other occupational. Leptospires usually live in the renal tubules of the kidneys or in tissues and organ of carriers. The incidences occurred in warm or tropical regions rather than areas of the temperate zone (Leptospirosis- a zoonotic disease of global importance) due to longer survival rates in the warm environment and humid conditions. The optimum growth temperature is about 28-30 0C. In Thailand the most of leptospirosis cases occur in northern and northeastern region. |
2. ARIMA Model
The time series data is analyzed and fitted by using Autoregressive Integrated Moving Average, ARIMA(p, d, q)x(P, D, Q)s model, which are the most general class of models for fitting and forecasting time series data. ARIMA models consists of 3 parts a) Auto regression model, b) Moving average model, and c) differencing order (Chatfield, 2000; Cryer and Chan, 2008), where:
- p and P are the auto regressive (AR) order and seasonal order of AR, respectively; - d and D are the differencing order and seasonal differencing order, respectively;
- q and Q are the moving average (MA) order and seasonal moving average order, respectively;
- s is the seasonal period.
For applying ARIMA models
1. we first have to check the time series data by autocorrelation function (ACF) plot. If the time series is stationary, the ACF plot will show the fluctuated pattern. But if time series is non-stationary, we have to remove the non-stationary term, by using log transform and/or differencing order, then re-test the stationary character of the time series by ACF plot, and Dickey-Fuller test (p-value < 0.01).
2. We then find the p and q orders by using cut-off time lag of ACF and partial autocorrelation function (PACF) plots. We first tested the numerous different values of p and q orders for fitting non-seasonal univariate ARIMA model. The coefficients of non-seasonal univariate ARIMA model are estimated by using mean square method, which assists in the goodness of fit process. The residuals of model results are checked for independence of the noise term through ACF and PACF plots. If the residuals have nearly white noise, the goodness of fit is examined through calculated Akaike’s Information Criterion (AIC) and root mean square error (RMSE).
3. After that we next find the seasonal univeriate ARIMA models. We first removed the trend and seasonal components from the data series, after which we carried out the same procedure as described above to find the P and Q orders. The ARIMA model is tested to find the best-fitted model and then use that model to predict the next leptospirosis season.
4. In order to investigate the climate factor, we incorporated climate variables as input series in the ARIMA models, called ARIMAX models. We calculated the cross-correlations function (CCF) between the pre-whitened climate series with leptospirosis cases series to find significant time lags at p-value < 0.05.
5. Similar to the univariate ARIMA models, we estimated the coefficients of ARIMAX models associated with the lagged climate variable. We tested the different time lags of climate input one at a time before combining them together. The ARIMAX models are re-fitted and used to predict future leptospirosis season.
|
3.Results:Northern Region
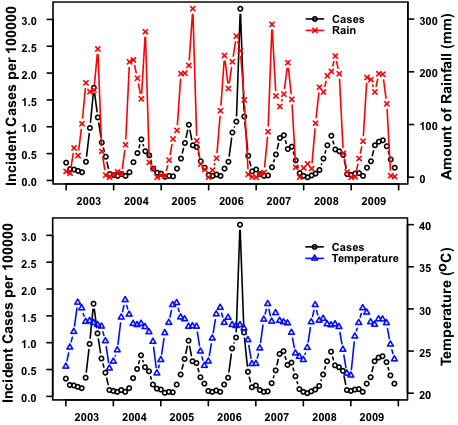
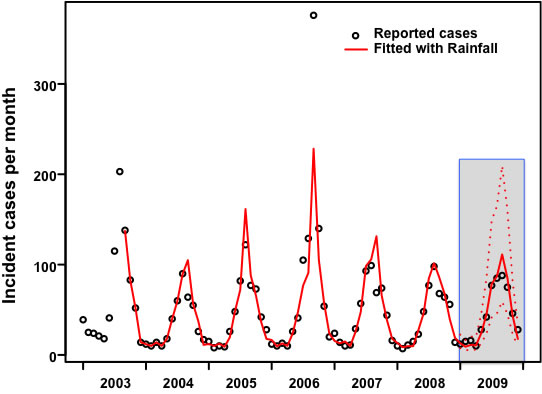
3.Results:Northeastern Region
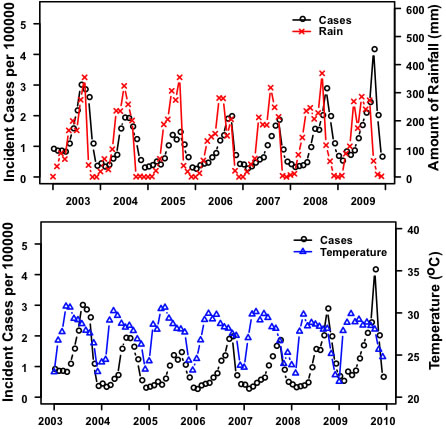
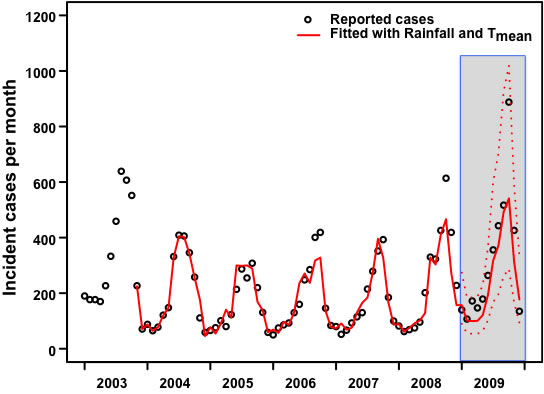
|
| 4.Sample Code |
5.References
1.Chadsuthi, S., Modchang, C., Lenbury, Y., Iamsirithaworn, S., Triampo, W., 2012. Modeling seasonal leptospirosis transmission and its association with rainfall and temperature in Thailand using time-series and ARIMAX analyses. Asian Pac J Trop Med 5, 539-546.
2.Cryer, J.D., Chan, K.-S., 2008. Time Series Analysis: With Applications in R, 2 ed. Springer Science+Business Media, New York, USA.
3.Levett, P.N., 2001. Leptospirosis. Clin Microbiol Rev 14, 296-326.
4.Bureau of Epidemiology DoDC, Ministry of Public Health, Thailand. National notifiable disease surveillance (Report 506). 2003-2009; http://www.boe.moph.go.th/boedb/surdata/disease.php?ds=43. Accessed September, 14, 2011. |
|
|