Cell Membrane by Mr.Kan Sornbundit
Biological membranes are very complex systems (see Fig.1) because it contains up to 500 different lipids species such as spingomyelins, glycerophospholipids and cholesterols.
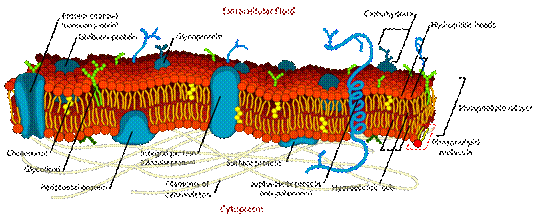
Figure 1. An illustration of biological membranes.
These species can be characterized into three major classes of lipid: phospholipids, glycolipids and sterols. Phospholipids are the major component of cell membrane. It consists of hydrophilic, like water, head group which has negatively charge, and two hydrophobic, hate water, fatty acid hydrocarbon chains. Phospholipids can be divided by character of hydrocarbon tails. Long-chain hydrocarbon tails are defined as saturated phospholipids while unsaturated phospholipids have shorter and kinked hydrocarbon chain. Glycolipids are phospholipids attracted with carbohydrate. The other important component in cell membranes is cholesterols, the well-known sterols. The basic structure of biological membranes is two phospholipids sheets that all fatty-acid tails point to the center of the sheet. Hydrophilic heads of outer layer, called cytoplasmic leaflet, are point to environment whereas hydrophilic heads of inner layer are point to cytoplasm.
In 1997, K. Simons and E. Ikonen [1] proposed that biological membranes are inhomogeneous because some lipids are laterally organized into nano-scale regions, called lipid rafts, distribute on both monolayer (see Fig.2). The recent lipid rafts definition state that lipid rafts are very small (10-200 nm), heterogeneous, highly dynamic, sterol- and sphingolipid- enriched domain [2]. Therefore, we can indicate lipid rafts as saturated phospholipid and cholesterol-containing regions that depleted from the cholesterol-poor or unsaturated phospholipid regions. In the present, we have believed that lipid rafts involve many biological functions such as signaling, recruitment of specific proteins and endocytosis. With this point of view, biological membranes are not only cell barrier but also behave like a platform of biochemical reactions.
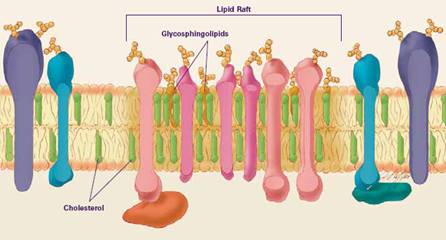
Figure 2. An illustration of a lipid raft on a biological membrane.
- Phase Separation On Simple membranes
Studying phase separation usually performs on simple membranes because it easily to control lipid compositions and can provide micron-size in cholesterol-containing domains, larger than typical size of lipid rafts. Simple membrane (or vesicle) is composed of two-opposed phospholipids monolayer and contains only three components: saturated phospholipids, unsaturated phospholipids and cholesterols. These components mimic the major types of lipid components in biological membranes. Now, we have to note that the word lipid raft is reserved for cholesterol-containing phases in biological membranes. Therefore, if we refer to cholesterol-containing domains in simple membranes, we must use the new word. The commonly used terms for cholesterol-containing phases in model membranes are liquid-ordered 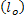 phase and unsaturated phospholipids phase is called liquid-disordered phase and unsaturated phospholipids phase is called liquid-disordered 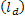 phase. phase.
In 2002, S. L. Veatch and S. Keller [3] performed experiments on symmetric ternary lipid bilayers, which both layers have the same lipid compositions. They found that  domains on both layers are completely in registration, no overlapping regions are observed. In 2008, M. D. Collins and S. L. Keller [4] observed domain formations in asymmetric planar lipid bilayers. They found that varying lipid composition in one monolayer can induce or suppress domain formations in the other monolayer. It leads consequently to the crucial hypothesis: there is coupling or strong inter-layer interaction. domains on both layers are completely in registration, no overlapping regions are observed. In 2008, M. D. Collins and S. L. Keller [4] observed domain formations in asymmetric planar lipid bilayers. They found that varying lipid composition in one monolayer can induce or suppress domain formations in the other monolayer. It leads consequently to the crucial hypothesis: there is coupling or strong inter-layer interaction.
- Growth law of lipid domains
One problem of domain formations in simple membranes is finding the growth law of  domains. Some previous works for this problem are reviewed as follow domains. Some previous works for this problem are reviewed as follow
4.1 Experimental work
In 2007, Yanagisawa et. al. [5] measured the growth law of ternary fluid vesicle which is composed of DPPC (dipalmitoylphosphatidylcholine), DOPC (dioleoylphosphatidylcholine) and cholesterol. They use fluorescence microscopy and image analysis to extract the growth law. They reported that there are two coarsening processes, normal and trapped coarsening. For normal coarsening, domains grow in diffusion-and-coalescence manner while domains are suppressed at some domain sizes. The growth law for normal coarsening was found to be 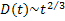 , where , where 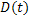 is a mean domain diameter. is a mean domain diameter.
4.2 Theoretical work
The starting point for this approach is to define the appropriate model for simple membrane. The model should capture the realistic structure of the simple membrane as much as possible.
The computational work of Gomez and coworkers [6], in 2009, proposed a 2-D Ising model based lipid bilayer model containing three components: saturated phospholipids, unsaturated phospholipids and cholesterol. They modeled lipid bilayers as two interconnected layers: a triangular lattice and a hexagonal lattice. In their model, species A, B and C refer to saturated lipids, unsaturated lipids and cholesterol, respectively. The species A and B are fully located on the triangular lattice while the hexagonal lattice is not fully occupied by C. They considered nearest-neighbor interactions between A and B while the interaction between A and C represents the coupling between two layers. They simulated the systems using Monte Carlo method with Kawasaki dynamics and enhanced bulk diffusion (EBD) dynamics - an algorithm that reduces the number of MC steps to complete the transient surface diffusion. Their results give the growth law for all species based on the EBD algorithm according to 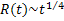 for early stages and the late stages is for early stages and the late stages is 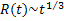 but the growth law from the Kawasaki algorithm cannot be observed. but the growth law from the Kawasaki algorithm cannot be observed.
Now, the growth law of domain is still discussed. Our group also interested in this problem. Finding the growth law from Monte Carlo method is in progress.
References
1. Simons, K. and E. Ikonen, Functional rafts in cell membranes. Nature, 1997. 387(6633): p. 569-572.
2. Pike, L., Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. The Journal of Lipid Research, 2006. 47(7): p. 1597.
3. Veatch, S.L. and S.L. Keller, Organization in Lipid Membranes Containing Cholesterol. Physical Review Letters, 2002. 89(26): p. 268101.
4. Collins, M.D. and S.L. Keller, Tuning lipid mixtures to induce or suppress domain formation across leaflets of unsupported asymmetric bilayers. Proceedings of the National Academy of Sciences of the United States of America, 2008. 105(1): p. 124-128.
5. Yanagisawa, M., et al., Growth dynamics of domains in ternary fluid vesicles. Biophysical journal, 2007. 92(1): p. 115-125.
6. Gรณmez, J., F. Sagรบs, and R. Reigada, Use of an enhanced bulk diffusion-based algorithm for phase separation of a ternary mixture. Journal of Chemical Physics, 2008. 129(18).
---------------------------------------------------------------------------------------------------------- |